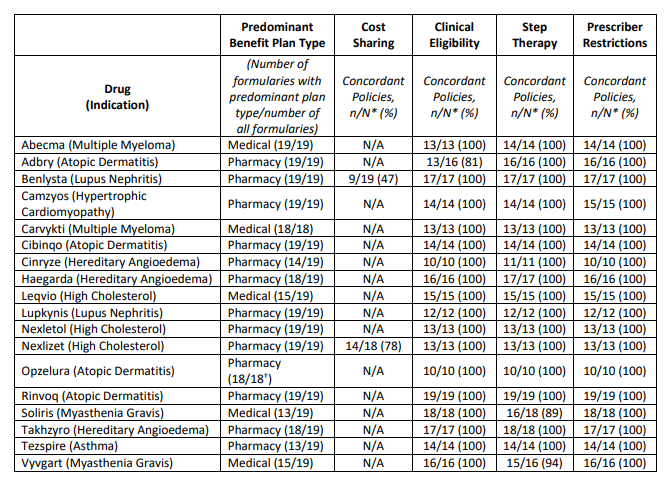
Last month, ICER released its 2023 “Evaluation of barriers to fair access” . The report concludes the following regarding 18 medications evaluated.
ICER defines “fair access” based on the following criteria:
Buy costs
- Cost sharing based on net price. Patient cost sharing should be based on the net price to the plan sponsor, not the non-negotiated list price.
- No cost for high-value therapies.. All medications identified by the Internal Revenue Service as high-value therapies must receive pre-deductible coverage within high-deductible health plans.
- A low-cost option available in each class.. At least one drug in each class must be covered at the lowest relevant cost-sharing tier, unless all drugs are priced above the established fair value threshold.
- It’s okay to have high cost sharing if no drug is profitable. If all drugs in a class are priced such that there is no single drug that represents fair value as determined by value assessment, it is reasonable for payers to have all drugs in a higher cost-sharing tier.
- If all drugs are priced fairly, formulary placement is acceptable. If all drugs in a class are priced to represent fair value, it remains reasonable for payers to use preferred formulary placement with tiered cost sharing to help achieve lower overall costs.
- Limited cost sharing if required step by step. As part of affordable step therapy, when patients try a lower-cost option at a lower cost-sharing level but do not achieve an adequate clinical response, cost-sharing for additional therapies must also be at the lower cost-sharing level, as long as those additional therapies are priced fairly according to transparent criteria.
Although ICER lists six criteria, only three (3, 4, and 5) are formally evaluated in its report.
Clinical Eligibility
- Payers should offer alternatives to prior authorization protocols, such as programs that provide feedback on prescribing patterns to physicians or waive prior authorization (“gold card”) requirements if they demonstrate high fidelity to evidence-based prescribing. .
- Payers must document at least annually that clinical eligibility criteria are based on current, high-quality evidence, with input from physicians with expertise in the same or similar clinical specialty.
- Clinical eligibility criteria should be developed with explicit mechanisms that require payer staff to document that they have: (i) considered evidence limitations due to systemic underrepresentation of minority populations; and (ii) sought input from clinical experts on whether there are distinctive benefits and harms of treatment that may arise for biological, cultural, or social reasons in different communities; and (iii) confirmed that the clinical eligibility criteria have not gone beyond the reasonable use of clinical trial inclusion/exclusion criteria to interpret or restrict FDA label language in a way that harms patients with underlying disabilities unrelated to the condition being treated.
- For all drugs: Clinical eligibility criteria that supplement FDA label language may be used to: (i) establish standards for diagnosis; and/or • Define indeterminate clinical terms in the FDA label (e.g., “moderate to severe”) with explicit reference to clinical guidelines or other standards; and/or (ii) triage patients based on clinical acuity when the payer explicitly documents that triage is reasonable and necessary
- For drugs with prices or price increases that have been deemed reasonable: Except for the three purposes described above, clinical eligibility criteria must not deviate from FDA label language in a way that reduces coverage.
- For drugs with prices or price increases that have been deemed reasonable: Documentation that patients meet clinical eligibility criteria should pose a light administrative burden, including acceptance of physician certification in lieu of more medical record documentation. formal, unless documentation is essential to ensure patient safety.
- For drugs with prices or price increases that have been deemed unreasonable: Clinical eligibility criteria may limit coverage by applying specific eligibility criteria to pivotal trials used to generate evidence for FDA approval if implemented with a reasonable flexibility and are supported by robust appeal procedures as described in the implementation criteria.
Step therapy and change
- To justify step therapy economic policies that extend beyond FDA labeling, as appropriate, payers must explicitly state or present evidence to document all of the following: • Use of first-step therapy reduces overall spending on medical care, not just spending on medications.
- First-step therapy is clinically appropriate for all or nearly all patients and poses no increased risk of significant side effects or harm.
- Patients will have a reasonable chance of achieving their clinical goals with first-step therapy.
- Failure of the first-step agent and the resulting delay in initiation of the second-step agent will not result in long-term harm to patients.
- Patients are not required to retry a first-line medication with which they have previously had adverse side effects or an inadequate response at a reasonable dose and duration.
- To justify required change policies as applicable, payers must explicitly state or present evidence to document all of the following: (i) Use of the required medication reduces overall health care spending. (ii) the required switch therapy is based on the same mechanism of action or has a comparable risk and side effect profile as the index therapy. (iii) the required switch therapy has the same route of administration or the difference in the route of administration will not create a significant negative impact on patients due to clinical or socioeconomic factors. and (iv) patients are not required to switch to a medication they have previously used at a reasonable dose and duration with inadequate response and/or significant side effects, including prior use with a different payer.
Supplier Qualifications
- Restrictions on coverage for specialized prescribers are reasonable with one or more of the following justifications: Ii) accurate diagnosis and prescription require specialized training, with the risk that non-specialist physicians will prescribe the drug to patients who may be harmed or poorly they are likely to benefit. (ii) Determining the risks and benefits of treatment for individual patients requires specialized training due to the potential for serious side effects of the therapy. (iii) dosing, monitoring for side effects, and overall coordination of care require specialized training to ensure safe and effective use of the medication.
- Requiring non-specialist doctors to certify that they are seeing the patient in consultation with a relevant specialist is a reasonable option when the condition is frequently treated in primary care settings, but some elements of dosing, monitoring for side effects and/or general coordination of treatment are necessary. Care would benefit from specialist input for many patients.
Fair access criteria
- Cost-sharing policies should be clearly presented to consumers before selecting a health plan, allowing all people to understand what cost-sharing they will face for treatments they are currently taking or considering.
- Any significant change in formulary or cost-sharing structures should not occur mid-cycle unless plan sponsors include it as a qualifying event that allows plan enrollees to change plans.
- At the point of care, physicians and patients should be able to quickly determine cost-sharing requirements for any treatment along with cost-sharing for other alternatives.
- People considering enrolling in a health plan should be presented with clear information that allows them to understand whether they meet insurers’ clinical criteria for the treatments they are currently taking. Policies must also set out the rationale behind them and be easily understandable.
- Clinicians and patients should be able to quickly determine the clinical criteria for any treatment and see the clinical rationale supporting these criteria. The referenced clinical information must be readily available to the prescribing/ordering provider and to the public.
- People considering enrolling in a health plan should be presented with clear information that allows them to understand whether the treatments they are currently taking or plan to take will be subject to non-medical step therapy or change policies.
- Physicians, pharmacists, and patients should be able to quickly determine requirements related to step therapy and policy change and be able to easily see a full insurer justification.
- People considering enrolling in a health plan should be able to easily find information related to coverage criteria, including prescriber qualifications, for medications they or their family members are currently taking.
- Doctors and patients should be able to quickly determine if there is a restriction on prescribing any treatment. Insurers should provide immediate assistance to primary care physicians seeking to connect with a relevant specialist for consultations as needed.
You can read the full report here.








